What is sterile filtration?
2025-04-08
MS
92
Sterile filtration is a process used to remove microorganisms, such as bacteria, viruses, and fungi, from a liquid or gas to ensure that the product is free from contaminants. This is typically achieved using filters with very small pore sizes, often ranging from 0.1 to 0.45 microns, which are small enough to capture even tiny microbial organisms while allowing the liquid or gas to pass through.
Sterilizing filters are used throughout pharmaceuticals, biotechnology, food and beverages, and healthcare to control bio-burden by removing bacterial contaminants. It is often applied to solutions that can't be sterilized by heat, irradiation, or chemicals. The goal is to maintain the safety and quality of the product while ensuring it remains free from harmful microorganisms.
How does sterile filtration work?
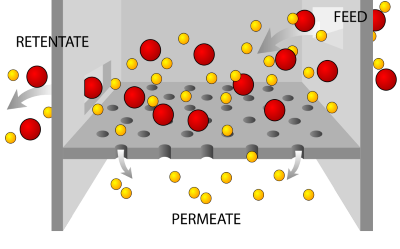
The filtration sterilization process uses porous membrane filters with membranes such as nylon, polyvinylidene fluoride (PVDF), or polyethersulfone (PES). The pores in the membranes are of differing sizes that prevent any substance larger than the size of the pore from passing through. The smaller the pore, the more effective the filter is at preventing substances from going through to the other side of the filter. In the diagram, the larger, red particles are not able to pass through the pores in the filter. The smaller, yellow particles, however, pass through to the other side of the filter.
How to identify the sterilization performance of a filter element?
Sterilizing filters should be validated using test procedures that comply with ASTM F 838-15(ae1) protocols for the determination of bacterial retention in filters used for liquid filtration. The challenge level is a minimum of 107 organisms per cm2 of filter media. CPF filters have > 7-log removal when challenged with the organisms listed below (0.03μm, 0.10μm, and 0.22μm meet the FDA definition of sterilizing grade filters).
|
Pore Size |
Organism Used to Validate |
|
0.03μm |
Acholeplasma laidlawii |
|
0.10μm |
Brevundimonas diminuta |
|
0.22μm |
Brevundimonas diminuta |
How to choose the right sterile filter?
- Level of Sterilization
The first and most important factor is what level of sterilization is required. Are you looking to remove bacteria only? Is mycoplasma removal required? Is endotoxin contamination a concern? The answers to these questions will determine the pore size and filter type required for your application.
- bacteria bioburden 300Basic bacteria removal can be achieved with a 0.2 or 0.22 micron (different manufacturers tend to use these ratings interchangeably - the most important factor is that it is validated for bacteria retention).
- MycoplasmaFor mycoplasma removal, a smaller pore size is required - 0.1 or even 0.03 microns depending on the manufacturer and validation testing.
- Endotoxin large 800Where endotoxin removal is also required, a positively charged version of these filters is needed.
- Fluid Characteristics
The chemical composition of your process fluid may limit the choice of membrane materials. For aqueous solutions, Polyethersulfone is usually the best choice due to its high flow rate and dirt-holding capacity. For other fluids that may negatively affect PES, Nylon 6,6 membrane would be a better choice.
- Flow Rate, Batch Size, Filter Longevity
Adding an integrated prefilter will reduce the flow rate per cartridge, but the trade-off could be worth it if service life is extended to meet batch size requirements. The reduced flow can also be offset by using a longer cartridge or capsule - 20” or 30” instead of a basic 10” - or multiple cartridges in the same housing.
4. Filter Quality & Materials of Construction
Materials used to manufacture sterilizing filters must be carefully selected and controlled to ensure they meet applicable quality and regulatory requirements. All materials must be non-toxic and meet USP Class VI and other toxicology requirements. In addition, the filters must not contain extract-able substances that could alter or contaminate the fluid being filtered. This includes proper selection of raw materials, manufacturing equipment, and area cleanliness to protect the filters from the surrounding environment.
Membrane Solutions Sterilizing Filter Options
|
Membrane Materials |
Filter Cartridges choices |
|
PES |
SteriPure-SPB for cost-effective and high flow sterilization filtration |
|
SteriPure-SPH with highly asymmetric PES membrane |
|
|
SteriPure-SPP/SPD double layer PES membrane for longer service life |
|
|
PVDF |
KynarPure-L for low protein binding sterilization filtration |
|
Nylon 66 |
PolymidPure Nylon 66 for sterilization filtration |
|
Positively charged Nylon 66 |
MinyPure-NZ Charged Nylon 66 for endotonxin removal |
|
PTFE |
FluorPure-SG ank vent and process gas filtration. |