What is Reverse Osmosis membrane?
2025-04-07
MS
46
A Reverse Osmosis (RO) membrane is a specialized semi-permeable barrier used in water purification systems. It plays a critical role by allowing water molecules to pass through while blocking the majority of dissolved salts, impurities, and contaminants. This article explores how a Reverse Osmosis membrane functions, detailing its importance in the RO process and its various applications. Aimed at readers with little to no experience in water filtration, this guide provides a simplified explanation of the role of the membrane in both consumer and industrial water treatment systems.
1.Understanding Osmosis and Reverse Osmosis
To understand reverse osmosis membrane, we must first understand osmosis and reverse osmosis.
Reverse Osmosis (RO) operates by forcing water through a semi-permeable membrane to eliminate a substantial proportion of dissolved solids and contaminants.
Osmosis
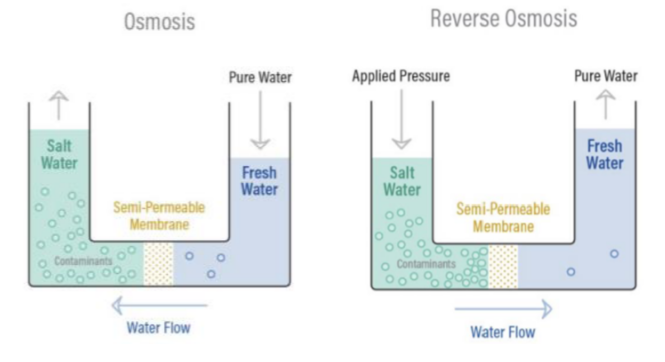
To fully grasp RO, it is important to first understand the natural process of osmosis. Osmosis is a biological and physical phenomenon where water moves from a lower concentration of solutes to a higher concentration, such as when plant roots absorb water from the soil or when our kidneys filter water from the bloodstream.
The diagram on the right illustrates how osmosis and Reverse Osmosis function. In this natural process, a less concentrated solution tends to migrate toward a more concentrated one.
For instance, if you place a container of water with low salt concentration next to another container with high salt concentration, separated by a semi-permeable membrane, the water will naturally flow from the lower to the higher concentration.
A semi-permeable membrane is one that permits certain molecules or atoms to pass through while blocking others. A common example is a screen door, which lets air pass but prevents pests. Similarly, specialized fabrics like Gore-Tex allow water vapor to pass while blocking liquid water.
The diagram below shows how osmosis and Reverse Osmosis works.
Reverse Osmosis
RO is the process of Osmosis in reverse. Osmosis occurs naturally without an external energy source, but reversing the osmosis process requires applying energy to the more saline solution to reverse the natural flow.
A reverse osmosis membrane is a semi-permeable membrane that allows the passage of water molecules but not most of the dissolved salts, organics, bacteria, and pyrogens. However, the water must be “pushed” through the RO membrane by applying pressure greater than the naturally occurring osmotic pressure.When pressure is applied to the concentrated solution, the water molecules are forced through the semi- permeable membrane while the contaminants are not allowed through.
2.How does Reverse Osmosis membrane work?
RO systems use a high-pressure pump to apply force to the feed water, pushing it across a semi-permeable membrane. This action effectively removes 95% to 99% of dissolved salts, leaving the unwanted salts and contaminants in the reject stream.
The amount of pressure required depends on the concentration of salts in the feed water. The more concentrated the water, the greater the pressure needed to overcome osmotic pressure.
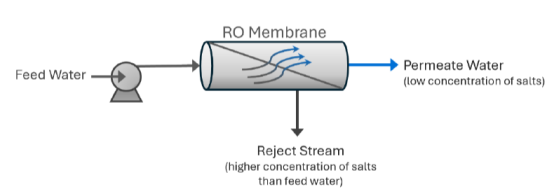
Permeate vs. Concentrate Water
In simple terms, two types of water flow through an RO system: the purified water, known as permeate, and the waste water, called concentrate. The permeate is the water that has passed through the membrane after most of the contaminants have been removed. For instance, a 100-gallon per minute (gpm) RO system will produce 100 gpm of permeate water.
The concentrate, sometimes referred to as reject or brine, is the water left behind with contaminants that couldn't pass through the membrane. This concentrate either goes to waste, or in some cases, can be redirected into the feed water supply for recycling purposes.
RO systems use cross-flow filtration instead of traditional dead-end filtration. In cross-flow filtration, water flows parallel to the membrane, and contaminants are continuously flushed away. This method minimizes build-up and keeps the membrane cleaner, allowing more efficient filtration.
3.What Contaminants will Reverse Osmosis membrane remove from water?
RO is capable of eliminating up to 99% of dissolved salts (ions), colloids, organic matter, bacteria, and pyrogens from water. The membrane rejects contaminants based on their size and charge. Molecules with a molecular weight greater than 200 are typically filtered out by the RO system.
The ionic charge of contaminants also plays a role in how effectively they are removed. For instance, calcium, which has two charges, is rejected more easily than sodium, which only has one charge.
However, it is important to note that RO systems do not efficiently remove dissolved gases like carbon dioxide (CO2), as these gases have a low molecular weight and are not highly ionized. As a result, the pH of the permeate water may be slightly lower due to the formation of carbonic acid when CO2 dissolves in water.
RO is particularly effective for treating brackish, surface, and groundwater, serving applications in a wide range of industries such as pharmaceuticals, food and beverage production, metal finishing, and semiconductor manufacturing.
Conclusion
At Membrane Solutions®, we are dedicated to providing advanced water purification solutions that meet the diverse and growing needs of industries and consumers. Reverse Osmosis (RO) technology is at the heart of our offerings, ensuring clean, high-quality water for a variety of applications. While RO cannot remove dissolved gases such as CO2, its ability to filter out salts, bacteria, and other harmful contaminants makes it a crucial tool for producing purified water.
Explore how Membrane Solutions® can support your water purification requirements with state-of-the-art RO systems designed for both large-scale industrial use and everyday applications. Visit our website at www.zsxmh.com to learn more about our products and how we can assist you with your specific needs.