How to Choose Biotechnology Filtration Products?
2025-04-14
MS
39
What is Biotechnology Filtration?
Filtration is the process of removing solid particles from a liquid or gaseous fluid by using a filter medium that allows the liquid to pass through but retains solid particles. A clarified liquid or the solid particles removed from the liquid may be the desired product. In some chemical manufacturing processes, both the liquid filtrate and the solid filter cake are recovered. Other media such as electricity, light, and sound can also be filtered.
The fluid to be filtered will pass through the filter medium only when some driving force is applied. This force may be created by gravity, centrifugation, pressure applied to the liquid above the filter, or vacuum below the filter, or a combination of these forces. In large sand bed filters and simple laboratory filtration, only gravity may be used. A centrifuge with a porous filter medium chamber can be thought of as a filter in which the force of gravity is replaced by a centrifugal force many times greater than gravity. In difficult laboratory filtration situations, a partial vacuum is often applied to the container below the filter medium to increase the filtration rate. Most industrial filtration processes involve the use of pressure or vacuum (depending on the type of filter used) to increase the filtration rate and reduce the size of the equipment required.
Biotechnology filtration, also known as biopharmaceutical filtration, includes different filtration technologies used in various areas of the biopharmaceutical industry. These techniques are often used to purify or separate substances. Due to the different sizes of the substances to be filtered and the different properties of the different components in the mixture, the applicable filtration technology also varies. The most commonly used filtration technologies in the pharmaceutical industry include surface filtration, ultrafiltration, and depth filtration.
The Main Application of Biotechnology Filtration:
Biotechnology filtration is generally used in monoclonal antibodies, recombinant proteins, vaccines, blood, liquid products, gene therapy, cell therapy and other industries.
From upstream cell culture, downstream separation and purification, to terminal preparation filling, filtration solutions are involved.
Biotechnology is also widely used in the production of antibiotics and amino acids, clarification of fermentation broth and culture medium, sterilization of biological products, removal of pyrogens, enrichment of macromolecules such as polypeptide proteins, enzymes, cells, and viruses, and is also widely used in finished product concentration and purification, preparation of Chinese medicine extracts, and extraction of Chinese medicine. It is also used in process water, bottle washing water, oral liquid, injection water, pure water, ultrapure water, Chinese medicine injection, etc. in the pharmaceutical production process. It is widely used. Ensure the purity and quality of biological products in the production process, filter impurities such as microorganisms, viruses, cells, particles.
Which Biotechnology Filtration Products Does Membrane Solutions offer?
A. Sterile syringe filter
1. Sterilizing filtration of cell/bacterial cultures
2.Buffer sterilization filtration
3.Serum sterilization filtration
4.Liquid clarification
5.Sterilizing filtration of compound preparations
6.Filtration sterilization of bioactive substances that cannot be autoclaved
7.Sterilization and ventilation

- Vacuum filtration unit
Vacufil' Ready to use Disposable Vacuum Filtration units are
designed for sterile filtration of tissue culture media, biological
fluids, and buffers.Typically 250-{>}1000mL
Pre-sterilized
Includes membrane filter, clear polystyrene graduated funnel with
threaded polyethylene adapter and graduated polystyrene
reservoir with a separate polyethylene cap.
Optional integrated Glass fiber pre-filter to increase throughput
Funnel is designed with GL45 threads for use with various
reservoirs.
Whole map of related products:

How to Choose Suitable Biotechnology Filtration Products?
1. Characteristics of filtered samples
- Pore size
- Filtration volume
Characteristics of filtered samples:
A: Hydrophilic samples: It is suitable to use hydrophilic membranes, which are suitable for filtering solutions with water matrix. For example: tap water, surface water, and existing filter membranes are: water system membranes (mixed cellulose esters), nylon membranes (polyamide).
B: Organic and inorganic compound (alcohols, esters, oils) samples are suitable for nylon membranes (polyamide).
C: Protein solution samples: Suitable for filter membranes with low protein adsorption. That is, PVDF membranes.
D: Strong acid and strong alkali samples are suitable for hydrophobic PTFE (polytetrafluoroethylene membrane)
For common filter membranes, MS provides test tables for common solutions. If there is a matching requirement, you can send the detailed filter solution details and we can customize it for you. You can also contact sales to provide chemical compatibility reference tables
Pore size of membrane:
Pore size 0.45μm filter membrane: used for filtering the mobile phase of conventional samples, which can meet the general requirements of chromatographic analysis.
* Pore size 0.22μm filter membrane: can remove extremely fine particles in samples and mobile phases.
* Pore size 1-5μm filter membrane: generally used for turbid solutions that are difficult to handle, you can first use a 1-5μm filter membrane for pre-filtration, and then re-filter with the corresponding filter membrane
Filtration volume reference:
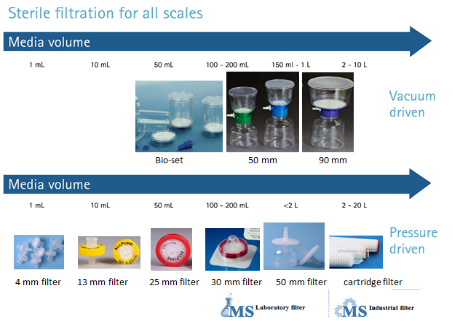
Commonly used membranes in biotechnology filtration and their characteristics:
PES membrane: Membrane Solutions PES membrane is produced by a unique preparation process: dry/wet/mixed method, with the characteristics of high flow rate/high flux/high pressure sterilization resistance/no adsorption of proteins and extracts/no pollution to samples. Pore size 0.02um/0.03um/0.05um/0.1um/0.22um/0.45um/0.65um/0.8um/1.0um/1.2/5.0um. The super asymmetric membrane pore structure has a higher porosity and has a higher water flux than other membranes (under the same pore size). The low protein adsorption characteristics of PES membrane make it very suitable for the preparation of biological samples.
PVDF membrane: Membrane Solutions provides hydrophilic PVDF membranes with various pore sizes (pore size range 0.05um-5um, width 254mm-304mm). At the same time, the company provides OEM customization for specific applications such as sterile filtration and biological sample preparation. The modified hydrophilic polyvinylidene fluoride (PVDF) membrane has ultra-low binding force, resistance to various chemicals and temperatures, high flow rate, and particle retention rate of {>} 99.99%. All types can be sterilized by high pressure and gamma ray sterilization. The binding force of biological molecules is low. It is the best choice for tissue culture medium, protein solution filtration, additive sterilization filtration, solvent and chemical raw material purification filtration, reagent aseptic processing, high-temperature liquid filtration and other applications.
What’s The Company Advantages of Membrane Solutions for Biotechnology Filtration Products?
Product research and development capability: With patented technology of independent research and development of filter membrane, independent research and development and production of Nylon, PVDF, PTFE, PES, etc.
Market validated product quality: After years of development, filter membrane and filter cartridge products have continued to provide stable and reliable filtration products for enterprises in different fields, including biopharmaceutical, medical devices, food and beverage, microelectronics, industrial filtration, and laboratory analysis.
The validation laboratory escorts the production process: the validation center has perfect management procedures, standard validation process and operation specifications, operates in accordance with the requirements of the ISO 9001 quality system, and establishes the management system and technical requirements of the validation center according to the requirements of CNAS-CL 01:2018 "Accreditation Standard for Testing and Calibration Laboratory Competence". The validation center consists of comprehensive microbial analysis laboratory, comprehensive chemical analysis laboratory, filtration performance laboratory and electron microscope analysis room, which provides complete testing and validation services for pharmaceutical enterprises and filter manufacturers, making the entire process more economical and reliable.
Welcome to know more about Membrane Solutions www.zsxmh.com

