Endotoxin Removal Membranes: The Guardian of Biotechnology Purification
2025-04-30
MS
2
Purity is of the utmost importance in the fields of biotechnology and pharmaceutical production. The presence of even trace amounts of contaminants can compromise the safety and efficacy of biological products. One such contaminant that poses a major threat is endotoxin, a toxic component of the outer membrane of Gram-negative bacteria. Endotoxins can cause strong immune responses that can be life-threatening in severe cases. To address this problem, scientists have developed a powerful tool: endotoxin removal membranes.
What is Endotoxin?
Endotoxins, also known as lipopolysaccharides (LPS), are complex molecules embedded in the outer membrane of certain bacteria. Unlike exotoxins secreted by bacteria, endotoxins are released when bacterial cells lyse or die. Endotoxins are heat stable and can withstand harsh environmental conditions, making them particularly difficult to remove. Endotoxin contamination is a significant issue in biopharmaceutical manufacturing because even trace amounts of endotoxins can cause adverse reactions in patients.
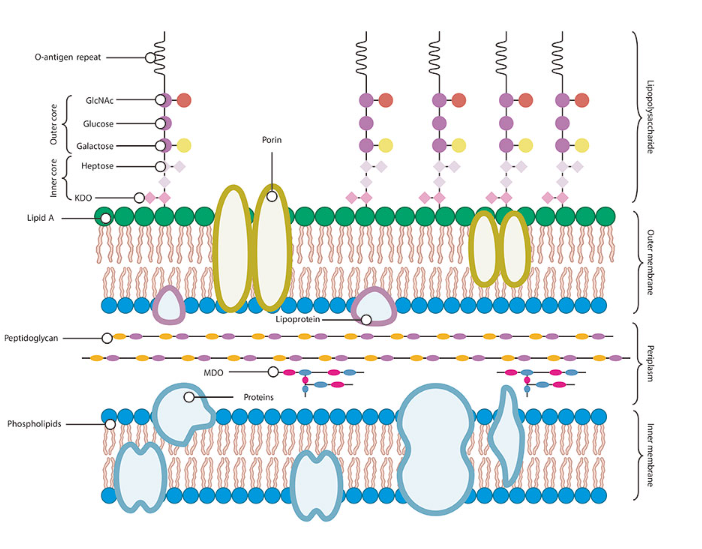
Composition of endotoxin
Necessity of endotoxin removal
Endotoxins in biological products such as vaccines, therapeutic proteins, and biopharmaceuticals can cause fever, inflammation, and even septic shock in recipients. Therefore, removing endotoxins or reducing them to acceptable levels is critical to ensure product safety and efficacy. Traditional endotoxin removal methods, such as heat treatment or chemical inactivation, may damage the desired biomolecules or fail to achieve the desired purity level. This is where endotoxin removal membranes come into play.
How Endotoxin Removal Membranes Work
Endotoxin removal membranes are specially designed filters that selectively retain endotoxins while allowing the passage of desired biomolecules. These membranes are typically made of polyethersulfone, nylon, or other synthetic materials and have a unique pore structure and surface chemistry that favors binding of endotoxins.
- The mechanism by which membranes remove endotoxins can be attributed to several factors:
Size Exclusion: Endotoxins are relatively large molecules compared to many biotherapeutics. Membranes with precisely controlled pore size can effectively retain endotoxins while allowing smaller therapeutic molecules to pass through.
Charge interaction: Endotoxins carry a negative charge, and membranes with positive charges on the surface can take advantage of this. The electrostatic attraction between negatively charged endotoxins and the positively charged membrane surface enhances the adsorption capacity of the membrane. The nylon positively charged membrane provided by MyBio uses this principle to remove endotoxins.
Hydrophobic interactions: Endotoxins also have hydrophobic properties and can be targeted by membranes with hydrophobic surface groups. These interactions further increase the efficiency of endotoxin removal.
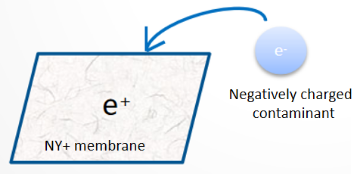
Schematic diagram of charge adsorption principle
Advantages of Endotoxin Removal Membranes
Endotoxin removal membranes offer several advantages over traditional purification methods:
Selectivity: They selectively remove endotoxins without affecting the integrity or activity of desired biomolecules.
Scalability: Membrane-based processes can be easily scaled up to large-scale biopharmaceutical production.
Cost-effectiveness: By reducing the need for extensive downstream purification steps, endotoxin removal membranes can reduce overall production costs.
Regulatory compliance: They help ensure compliance with regulatory standards for endotoxin levels in biologics.
In addition to the above advantages, the nylon positively charged membrane provided by Maiborui also has the advantages of high endotoxin adsorption capacity, excellent mechanical properties, chemical resistance, etc., and can be used for industrial use in filtering out endotoxins in biopharmaceuticals.
Virus removal pleated cartridge filter provided by Membrane Solutions
Biotechnology Applications
Endotoxin removal membranes are widely used in all stages of biopharmaceutical production, including upstream processing, downstream purification and final product formulation. They are particularly important in the production of parenteral therapeutics, where even trace amounts of endotoxins can pose a significant risk.
In addition to biopharmaceuticals, it can also be used in the production of medical devices, water purification systems, and other applications where endotoxin contamination must be minimized.
Conclusion
Endotoxin removal membranes are an essential component of the biotechnology purification process, providing a safe and effective method for removing endotoxins from biologics. The selectivity, scalability, and cost-effectiveness of endotoxin removal membranes make them an indispensable tool for ensuring the purity and safety of biopharmaceuticals. As biotechnology continues to advance, the development of more efficient and innovative endotoxin removal membranes will play a vital role in improving the quality and accessibility of life-saving therapies.

